
 |
Development |
sponsored by NRP53 |
|
||||
Setting-up new reviews can only be done by group coordinators (members of a "…-admin" group) for the coordinated group. Click New Review in the left menu.  Enter the title of the new review. Enter a description of the new review (optional). Select the appropriate review group (only the review goup(s) for which you are a coordinator are shown – ususally only one). Select whether the review will involve consensus for report identification and data extraction (usually yes). Press Create New Review button to confirm the creation of the new review (If you leave the New Review section without pressing the button all entered details will be lost and no new review will be created). 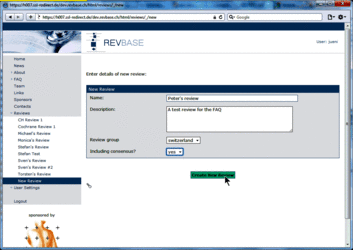 Go to Review Definition of the new review (either in the left menu or the overview table).  Add coordinator(s), author(s), and consentor(s) as described in the FAQ "How can I change user roles (coordinator, author, consentor) in a review?" below. Do not forget to confirm changes by pressing the Save button. Note: user rights described below apply only to the specific review for which a user was assigned the specific user role. Coordinator The review coordinator is the person for setting up the review and to coordinate the whole review process. The coordinator has the following rights in RevBase:
Author An author of a review is the person doing the literature screening, assessment, and data extraction. An author has the following rights in RevBase:
Consentor A consentor of a review is the person who does the various consensus in a systematic review i.e. the person who looks at the assessments/extractions done independently by two authors and who makes the final decision. Other names might be arbiter or adjudicator. A consentor has the following rights in RevBase:
Changing user roles for a specific review can only be done by group coordinators (members of a "…-admin" group). Go to Review Definition of the review for which you want to change user roles. A brief description of the different user roles and their assigned tasks is provided in the tool tips. 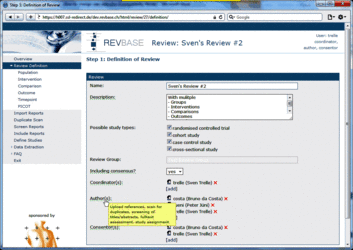 Adding a user to a specific role 1. Click [add] at the user role for which you want to add user(s) 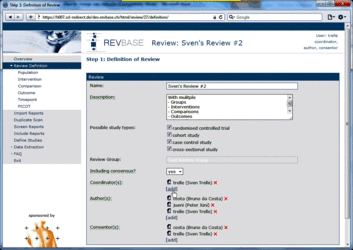 2. Select the user(s) you want to add to the specific user role 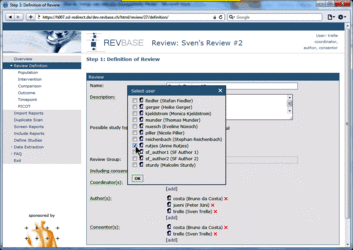 3. Press ok The added user(s) will be shown for the selected user role 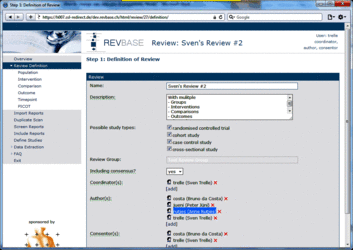 4. Press the Save button to confirm changes (otherwise all changes will be lost when exiting the Review Definition. 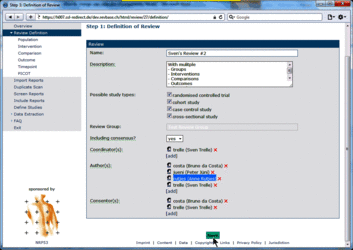 Retracting a user from a specific role 1. Click x at the user role from which you want to retract user(s) 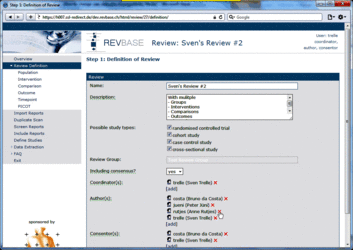 The retracted user(s) will not be shown for the specific user role 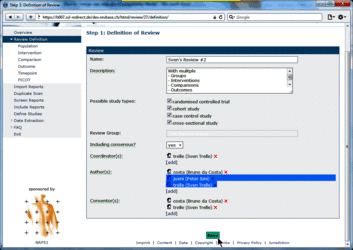 2. Press the Save button to confirm changes (otherwise all changes will be lost when exiting the Review Definition Data of component studies in a systematic review relates to what is called different levels of information in RevBase. For example, the number of study sites relates to the study level whereas the number of participants with missing outcome data might be different for each outcome measured at the various timepoints in various groups of participants (overall population or subgroups). Because RevBase has built-in core data extraction forms it is necessary to define these different levels of information explicitly. This is already done in the system. However, the user has to provide information on each of the levels relevant for a particular review in the "Definition of Review". The system will then use this information to label the different levels of information as appropriate. A particular PICOT in a systematic review is defined by the study population, the two interventions, the outcome and timepoint of measurement of interest. This concept is probably best explained by an example: Example A systematic review is looking at the effects of acetaminophen on headache. The authors defined in the protocol the following:
Yes, there are some restrictions although we believe that these will not be relevant in practice. The maximum numbers for the different levels of information that can be handled in RevBase are:
| ||||
![[+]](/public/pfeil_rechts.gif) About
About![[-]](/public/pfeil_unten.gif) FAQ
FAQ